1500 yuan a bottle, Merck Sharp & Dohme’s new crown capsules hundreds of thousands of boxes have arrived in Shanghai warehouses! Pfizer: will produce Paxlovid in China
After Pfizer Paxlovid, Henan real biological Azulfidine, the third in the domestic approved new crown oral drug Merzadone Monoravir capsules (Molnupiravir) China offer out.
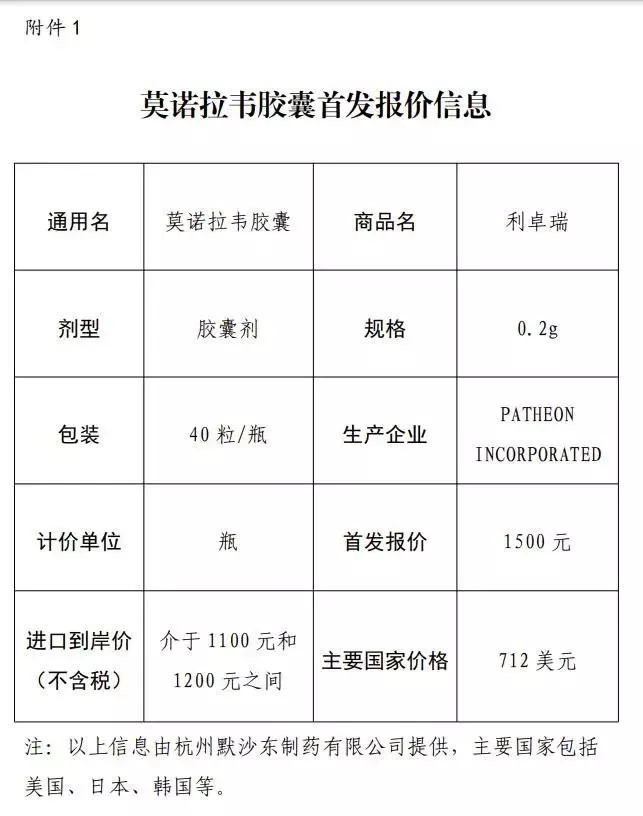
On January 10, Tianjin Pharmaceutical Procurement Center announced the public announcement of the first offer of the new crown treatment drug Molnupiravir capsule, the first offer of Molnupiravir capsule is 1500 RMB/bottle, 40 capsules/bottle, and the price of the drug in major countries is 712 USD.
Video Screenshot
The first offer of Merzadone’s new crown oral drug is $1,500 a bottle
The first few hundred thousand boxes have arrived at the Shanghai warehouse
On January 10, the Tianjin Pharmaceutical Procurement Center released the “Public Notice of the First Offer of the New Crown Therapeutic Drug Monoprevir Capsules”, which showed that the first offer of monoprevir capsules was 1500 RMB per bottle, 40 capsules/bottle, with the import c.i.f. price (excluding tax) between 1100 and 1200 RMB, and the price in major countries was 712 USD (about 4826 RMB), which was publicized from January 2023 10 to January 16.
The announcement said that recently, the Office of the National Health Security Bureau issued the “New Crown Therapeutic Drug Price Formation Guidelines (for trial implementation)”, and the innovative therapeutic drugs approved for listing from January 1, 2023 against the new crown virus take the first offer for centralized acceptance. Hangzhou Merck Sharp & Dohme Pharmaceutical Co., Ltd. took the initiative to refer to the relevant requirements and submitted the first offer and related information for the previously conditionally approved listing of Monoprevir capsules.
Data chart
Last December 29, the State Drug Administration in accordance with the relevant provisions of the Drug Administration Law, in accordance with the special drug approval procedures, emergency review and approval, conditional approval for the import registration of Merck Sharp & Dohme’s new coronavirus treatment drug, Monoprevir capsules (trade name: Lizoride / LAGEVRIO).
According to the official website of the FDA, this product is an oral small molecule neocoronavirus treatment drug for the treatment of adults with mild to moderate novel coronavirus infection (COVID-19) with high risk factors for progression to severe disease, such as patients with high risk factors for severe disease such as advanced age, obesity or overweight, chronic kidney disease, diabetes, severe cardiovascular disease, chronic obstructive pulmonary disease, and active cancer. Patients should use the drug strictly according to the instructions under the guidance of a physician.
The State Drug Administration requires the marketing license holder to continue to carry out relevant research work, complete the requirements of the attached conditions by the deadline, and submit the results of follow-up studies in a timely manner.
Initially, the price per vial of monoprevir capsules is quoted at a lower price than Pfizer’s new crown oral drug Paxlovid, which has a provisional medical insurance payment price of 1,890 yuan per box in some parts of China.
In September last year, Merck Sharp & Dohme signed a cooperation framework agreement with Sinopharm, granting the distribution and exclusive import rights of monoprevir in China to Sinopharm. In November last year, Mercer signed a distribution agreement with Sinopharm Distribution Center Ltd. for the import and distribution of monoprevir in China.
According to Punch News, Cai Baisong, vice president of Sinopharm Holdings, said in an interview on Jan. 10, “Optimistically, it is estimated that Mercer’s new crown oral drug, monoprevir, is expected to be sold in the domestic market before the Spring Festival.”
“At present, it is mainly formalities, operational level problems can be overcome.” For the latest progress of monoprevir into the domestic sales, Cai buy song to reporters, the early morning of January 4, the first batch of new crown oral drug monoprevir has arrived in Shanghai Waigaoqiao Free Trade Zone warehouse. “The number is in the hundreds of thousands of boxes, now arriving every day one after another, the follow-up will also continue to stock.”

File photo
Monoprevir capsules how effective?
Last December 29, the State Drug Administration in accordance with the relevant provisions of the Drug Administration Law, in accordance with the special approval procedures for drugs, emergency review and approval, with conditional approval for the import registration of Merzadone’s new coronavirus treatment drug Monoprevir capsules (trade name: Lizoride / LAGEVRIO).
On January 6, the National Health Commission released the “Treatment Plan for Novel Coronavirus Infection (Trial Version 10)” (hereinafter referred to as “Version 10”). Compared with the “Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 9)” released in March last year, the antiviral treatment includes the combination package of nematovir/ritonavir tablets (“Paxlovid”), monoclonal antibodies, COVID-19 human immunoglobulin and recovery plasma for recovered patients. In addition to Paxlovid, monoclonal antibodies, intravenous COVID-19 human immunoglobulin and plasma for recovering patients, the newest regimen includes the addition of monoprevir capsules.
In terms of the product use of the new crown drug, according to the 10th edition of the protocol, the population for which Monoprevir capsules are indicated is adult patients with mild to moderate disease within 5 days of onset and with high risk factors for progression to severe disease. It is administered as 800 mg orally every 12 hours for 5 days. It is not recommended for use during pregnancy and lactation.
According to Firstrade, Merck Sharp & Dohme’s Monoprevir capsules are approved for similar indications as Pfizer’s Paxlovid in adult patients with new coronavirus with risk factors for progression to severe disease, specifically Monoprevir capsules for the treatment of adults with mild to moderate novel coronavirus infection (COVID-19) with risk factors for progression to severe disease, such as advanced age, obesity or overweight, chronic kidney disease, diabetes mellitus, severe cardiovascular disease, and severe lactation. Patients with high risk factors for severe disease, such as advanced age, obesity or overweight, chronic kidney disease, diabetes, severe cardiovascular disease, chronic obstructive pulmonary disease, and active cancer.
However, Merck Sharp & Dohme Monoravir capsules have different targets than Pfizer Paxlovid has chosen to develop, as the former is an RNA polymerase (RdRp) inhibitor, which inhibits or clears the virus by blocking the synthesis of neo-coronavirus RNA-dependent RNA polymerase, while the latter is a 3CL protease inhibitor, which disrupts the virus by blocking the activity of neo-coronavirus 3CL protease, thereby disrupting the RNA replication process.
Data show that taking Paxlovid within 3 or 5 days of the onset of symptoms can reduce the risk of hospitalization or death associated with neointimal by up to 89% and 88%, and that monoprevir can reduce the risk of hospitalization/death by 30% to 50% and can reduce the risk of death by 89% to 100%.
Pfizer CEO: Paxlovid to be manufactured in China
Pfizer is working with a partner in China to begin local production of the new crown oral drug Paxlovid in China in the first half of this year, but the partner has not yet begun production and is expected to start soon, said Albert Bourla, chief executive officer of Pfizer, at the JPMorgan Healthcare Conference, Bloomberg reported on Jan. 9 local time.
Eberle did not disclose the name of the Chinese partner’s company and denied previous Reuters reports that some companies are producing and selling generic versions of Paxlovid in China.
According to the official website of the State Drug Administration, Paxlovid was approved for marketing in China in February last year, the first approved oral version of the new crown drug. On Dec. 14 last year, Pfizer announced that it signed a 2023 import distribution agreement with China Pharmaceuticals for the neo-crown virus treatment drug nematovir tablets/ritonavir tablets combination package (Paxlovid), and China Pharmaceuticals will be responsible for the import and distribution of Paxlovid in the mainland China market during the agreement period.
In an interview with CNBC on the 9th, Abreu said it is working to provide more Paxlovid to China, “We are working with China and we are trying to understand what their policies and needs are. Right now, they have a lot of interest in Paxlovid. Our production line is also working to secure supply for this phase.”
In August 2022, Zhejiang Huahai Pharmaceutical Co Ltd (Huahai Pharmaceutical, 600521) had signed a Manufacturing and Supply Master Agreement with Pfizer, under which Huahai Pharmaceutical would provide formulation commissioning services for Pfizer’s new coronavirus treatment Paxlovid, which is marketed in mainland China, for the duration of the agreement (5 years).
On the morning of January 9, Huahai Pharmaceuticals told the Punch News that the company is currently working with Pfizer to accelerate the work of the Paxlovid localization project to ensure adequate supply of Paxlovid in the Chinese market and continue to meet the demand for new coronavirus treatment for Chinese patients. When the reporter asked when localized production was expected to begin, the other party said, “It is recommended to consult Pfizer on this issue.”
Since the epidemic prevention and control measures were liberalized at the end of last year, demand for new crown treatment drugs, including Paxlovid, has surged, and Paxlovid is now available in community hospitals in some cities, prescribed by doctors for use. The price for entering the medical insurance is 1890 RMB/box (30 capsules in total).
It is worth noting that Paxlovid is out in the negotiation of the 2022 national health insurance catalog.
On January 8, the head of the National Health Insurance Bureau of Medicine Management Division in the introduction of new crown treatment drugs to participate in negotiations on the medical insurance drug catalog, said this year, a total of Azulfidine tablets, nematovir tablets / ritonavir tablets combination package (Paxlovid), lung detoxification particles 3 new crown treatment drugs through the enterprise independent declaration, form review, expert review and other procedures, to participate in the negotiations. Among them, Azulfidine tablets, lung detoxification granules negotiations were successful, Paxlovid because the manufacturer Pfizer Investment Co.


Average Rating