New crown generic drugs also have the risk of counterfeiting, the inflow of counterfeit drugs made in India into the Chinese market needs to be noted
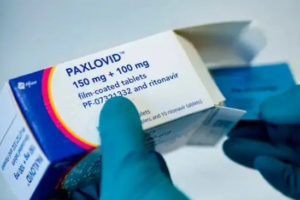
According to the official website of the National Health Insurance Administration of China on January 8, the “new crown drug” nematovir tablets/ritonavir tablets combination package (Paxlovid, also known as “Pfizer new crown drug”) was not successfully included in the medical insurance catalog due to the high price quoted by the manufacturer. The high cost and the high demand for the drug were not enough. The high cost and high demand have led some people to turn their attention to the generic Paxlovid.

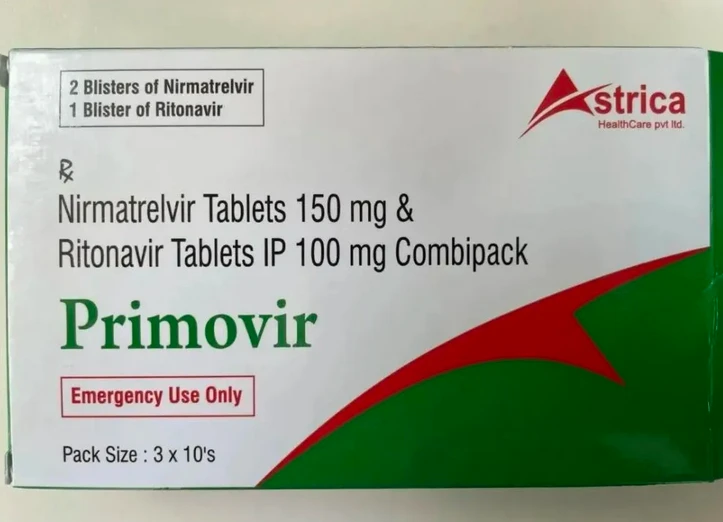
Green box generic Primovir box.
India has been known for its pharmaceutical industry and generic drug industry. Already, Indian media have focused on the circulation of Indian-made generic – and even “fake” – New Crown drugs in China. The Hindu reported on Jan. 9 that some “counterfeit” versions of India’s New Crown generics have found their way into the Chinese market because of the huge demand for New Crown drugs in Chinese society.
What is the origin of the Indian generic version of New Crown?
According to the U.S. media Quartz, on January 3, the WHO approved the application of a generic version of Nirmacom produced by the Indian pharmaceutical company Hetero on December 26 last year. This drug is generic to the original drug Paxlovid, which consists of nematovir and ritonavir. While an original drug is an original new drug, a generic drug is a drug with the same active ingredient, dosage form, route of administration and therapeutic effect as the original drug, manufactured under the authority of the original manufacturer to provide options for people in low- and middle-income countries.
This is the first time that a New Crown generic drug has been pre-qualified by the World Health Organization. Even though Paxlovid has been available in the U.S. for more than a year, its limited availability, delays in the approval process and high price still keep many low- and middle-income countries away from it, Quartz Financial Network noted.
According to a Jan. 9 report in the People’s Daily, the head of China’s National Health Insurance Bureau said the main reason for the unsuccessful negotiations to include Paxlovid in the Medicare program was the “high price quoted,” and that “although Paxlovid was not included in the Medicare catalog through negotiations, Medicare will still pay for it on a temporary basis until March 31, 2023.” This also means that after March 31 of this year, patients will have to pay out-of-pocket if they need to use Paxlovid.
The WHO’s approval of Nirmacom’s eligibility is expected to bring the Paxlovid generic onto formularies in 95 low- and middle-income countries, according to Quartz Finance. Hetero can produce generic versions of Paxlovid under the Medicines Patent Pool (MPP) agreement signed by Indian drug company Hetero, a public health organization supported by the United Nations that was originally established to provide HIV drugs to non-affluent countries. The MMP is a UN-backed public health organization that was initially used to provide HIV drugs to non-affluent countries, but later negotiated patent rights with large pharmaceutical companies to give manufacturers willing to sell affordable drugs to low- and middle-income countries the “non-exclusive right” to produce specific drugs.
In 2021, Pfizer agreed to offer generic rights to Paxlovid through the MMP without royalties. Since then, several pharmaceutical manufacturers around the world have been working to produce generic versions of the drug, and Hetero is only the first generic version of New Crown to receive WHO pre-approval, which means that quality is guaranteed during its production.
Previously, Nirmacom had already received emergency use approval in India. Another Indian pharmaceutical manufacturer Zenara pharma’s generic Paxlovid has also received emergency use approval in India. According to the prescription, Nirmacom should be started within five days of the onset of symptoms in patients with new crowns.
Hu Shanlian, a professor of health economics at Fudan University’s School of Public Health, said in an interview that Paxlovid’s pricing now does not include royalties, and that although the drug can be genericized, not every country can do so, with the U.S. delineating the range of countries where the drug can be genericized. China currently has five manufacturers who can genericize this drug, but cannot sell it domestically, “so we are now also suggesting whether we can agree to sell generic Paxlovid in the country on the basis of legal compliance, which is also our effort in terms of price.”
Generic drugs exposed as “fake”
The Indian New Crown generics that have appeared on Chinese e-commerce platforms include Primovir, Paxista, Molnunat and Molnatris, the first two copied by Paxlovid and the latter two copied by Molnipiravir, a joint venture between Merck Sharp & Dohme and Ricky Baker. The South China Morning Post reported earlier that Paxlovid costs about 2,980 yuan per box in China, while Indian generics can be bought for 530 to 1,600 yuan.
All four New Crown generics are approved by Indian authorities for emergency use, but it is not legal to use them in China. Indian media have stressed that India has been persuading China to allow the introduction of Indian pharmaceutical products because it would “reduce the huge trade deficit” between the two countries. Sahil Munjal, chairman of the Indian Pharmaceutical Export Promotion Council (Pharmexcil), told Reuters that India wants to strengthen the production of fever drugs and the export of these drugs to China.
But the Indian generic drug market is in disarray. The Times of India reported on Jan. 9 that some Chinese health experts have warned that the Chinese market is flooded with counterfeit versions of India’s new Crown generic drugs. Earlier reports indicated that some samples of generic Primovir did not contain nematovir, the main ingredient, meaning that it had no therapeutic effect and was a “fake drug.
Despite India’s proud pharmaceutical industry, the country’s society has been plagued by scandals of falsification and fraud regarding medical supplies for Neoguan since the outbreak of the epidemic. AFP reported in 2021 that Indian scams in areas such as drugs, oxygen cylinders, and protective equipment (PPE) have exploited the crisis caused by the New Crown pandemic for their own benefit, even with fatal consequences.
For example, some online scammers took advantage of the shortage of oxygen cylinders in Indian hospitals at the time to sell oxygen cylinders to people in need for as much as $205 (about $1,390), and refused to deliver after receiving the money, demanding more money from buyers. Other victims paid $616 for the purchase of oxygen cylinders, but could not get the goods at all. Other fraud rings have repainted fire extinguishers and sold them as oxygen cylinders.
A gang arrested by Indian authorities manufactured and sold a fake antiviral drug, Remdesivir, which cost about 20 Indian rupees ($1.65) per bottle and sold for more than 10,000 rupees ($823.55).
The G20 presidency continues to promote “medical diplomacy”?
Despite the chaos, the Indian authorities are still hoping to help developing countries with a new crown of domestically produced drugs, seeking to become the “voice of the global South. Indian Health Minister Mansukh Mandaviya stressed that India is the G20 presidency in 2023 and that addressing health emergencies, digital innovations for universal health coverage and strengthening the pharmaceutical sector are three priority areas for health during India’s G20 presidency, the Indian Express reported on Jan. 6.
According to The Indian Express, an unnamed Indian official said that the G20’s troika (the previous, current and next presidencies) are all developing countries and “it is the right time for India to raise the issues (facing) the global South. “As we saw during the new crown pandemic, even with the scaling up of vaccine production, developed countries are procuring several times more than they need, and some countries still don’t have (vaccines). We will call for equitable access for countries in the global South.”
In January, April, June and August of this year, India will hold meetings of health officials under the G20 framework. Indian authorities have stressed that the country will showcase its management of the new crown epidemic, health services, affordable responses and other matters at these meetings, and that India will use its pharmaceutical policy agenda to promote vaccine production, medicines and diagnostics “on-demand” globally.
In fact, as early as January 2021, India, known as the “world’s pharmaceutical company,” made a high-profile announcement that it would launch “vaccine diplomacy” to provide new vaccine crowns to developing countries such as its South Asian neighbors. According to information, the Serum Institute of India is currently the world’s largest vaccine producer, producing 1.5 billion doses of various vaccines annually before the outbreak of the new crown, 80% of which are exported.
Despite this, the Indian authorities’ “vaccine diplomacy” launched in January 2021 has raised questions about India’s ability to balance domestic vaccination. In the first half of 2021, India experienced a massive outbreak of new vaccines and failed to achieve its policy goal of curbing the epidemic by promoting vaccination in the country. At the time, the fragmentation and privatization of the country’s vaccine policy caused confusion, controversy, and criticism. As the epidemic situation in the country became tense, India’s plans to export vaccines overseas were put on hold, and the supply of vaccines to many countries around the world was affected.

Average Rating