
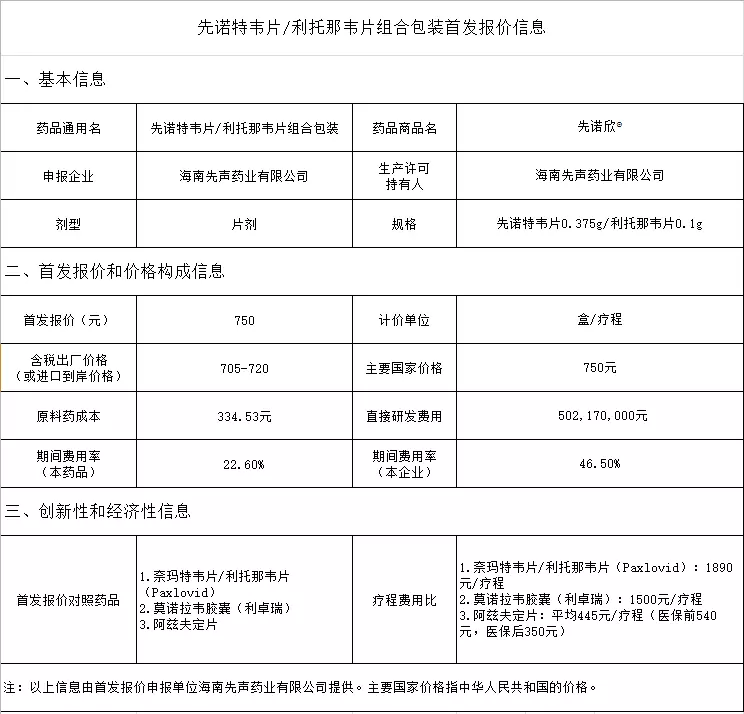
New crown therapeutic drug centronavir tablets/ritonavir tablets combination package first offer announcement

According to the “Office of the National Health Insurance Administration on the issuance of notice” (Medical Insurance Office issued [2023] No. 2) requirements, the Hainan Centrum Pharmaceutical Co., Ltd. cenotecanide tablets / ritonavir tablets combination package first offer information is now publicized (see Annex). Public notice period from January 31 to February 6, 2023, if there are objections, please submit the following ways to the Bureau of the real name during the public notice period, and provide legal and effective evidence materials (the name of the unit to be sealed). Failure to provide the corresponding evidence materials, in principle, will not be accepted.
- E-mail: [email protected]
- Letter: Beijing Municipal Medical Security Bureau Office, 11F, No.1 Xisanhuan South Road, Fengtai District, Beijing, 100071, and please indicate the word “public opinion” on the envelope.
- Fax: 010-89152514
Annex: 1. First offer of cenotecanavir tablets/ritonavir tablets combination package
- Clinical professional opinion of cenotecanavir tablets/ritonavir tablets combination package
- Centronavir tablet/ritonavir tablet combination package industry association recommendation
Beijing Municipal Medical Security Bureau
January 30, 2023
More Stories
Qingdao City, the new coronavirus nucleic acid testing “willing to detect as much as possible” sampling site list (as of February 3, 2023 9:00)
According to the deployment of the new coronavirus infection "B class B management", we will continue to provide nucleic acid...
Temporary transformation ward at the peak of the epidemic has been restored to its original function
At present, the overall neo-crown epidemic in China has entered a low epidemic level. In order to effectively respond to...
Those days as a nucleic acid sampler
▪ "I remember once I was in charge of checking the nucleic acid testing work, when a reagent tube slipped...
First domestic herpes zoster vaccine approved for marketing
On February 1, Bac Bio released the "Announcement on Voluntary Disclosure of the Approval of the Application for Marketing License...
CDC: peak of 1.625 million people infected with the new coronavirus in the hospital
On February 1, the Chinese Center for Disease Control and Prevention (CDC) reported the "national outbreak of novel coronavirus infection"....
Civil Aviation Administration: do a good job of checking the distant end of the foreign country, resolutely prevent the possible impact of the epidemic
CAAC held a teleconference on epidemic prevention and control According to the arrangement of the CAAC party group, on February...


Average Rating