
India’s new crown generic drug manufacturer is actually an import and export company, the Indian drug regulatory agencies official website search no such drug
On January 8, the much-anticipated Pfizer New Crown oral drug Paxlovid (nematovir tablets/ritonavir tablets) failed to enter the national medical insurance drug catalog due to high quotes, resulting in medical insurance negotiations. The company’s main goal is to provide the best possible solution to the problem.

▲Paxlovid generic Paxista (blue box).
The company has been in the process of developing a new product for the past few years. Is there any record information in the Indian drug regulatory authority? The reporter investigated this.
On January 9, the State Drug Administration responded that “drugs have to be approved by the State Drug Administration in order to legally carry out production and operation, it is advisable to buy through the hospital with a doctor’s prescription.”
Blue boxed Indian generic drug: booking 2000 yuan/box
Paxlovid, a new coronavirus treatment drug developed by Pfizer, was conditionally approved by the State Drug Administration for the treatment of adult patients with mild to moderate novel coronavirus pneumonia (COVID-19) with high risk factors for progression to severe disease in China on Feb. 11, 2022, according to the Daily Economic News, such as those with advanced age, chronic kidney disease, diabetes mellitus, cardiovascular disease, chronic lung disease, and other high risk factors for severe disease.
On November 16, 2021, Pfizer entered into a licensing agreement with the Medicinespatentpool (Mpp), a nonprofit organization, allowing it to further Pfizer has entered into a licensing agreement with the Medicinespatentpool (Mpp), a non-profit organization, allowing it to further license generic versions of Paxlovid to other pharmaceutical manufacturers. The company has also entered into a licensing agreement with the Medicinespatentpool, allowing it to further license other pharmaceutical companies to produce generic versions of Paxlovid.
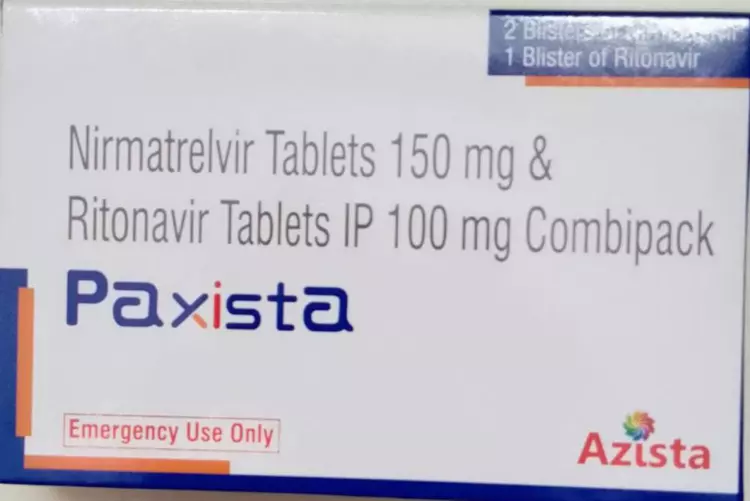
The generic version of Paxlovid, Paxista (blue box).
The company’s main business is to promote the development of the company’s products and services.
On January 9, a source told reporters, “The green box packaging Primovir now over not many people, the blue box packaging Paxista cost more than 1,000 yuan, selling about 2,000 yuan / box.”
Reporters noted that the blue box packaging Paxista by the Indian Azista (Azista) company production. So, the blue box packaging Paxista reliable? The above-mentioned informed source told reporters, “Information can only be verified online.”
The official website of the Indian drug regulatory agency to search no such drug National Drug Administration advice: buy the drug with a doctor’s prescription
The reporter saw in India Azista company official website, the company is Pfizer authorized generic Indian drug company Hetero (Hedron) sub-brand. The company’s official website describes Azista as a health care drug import and export company, whose main products are health care products and drugs.
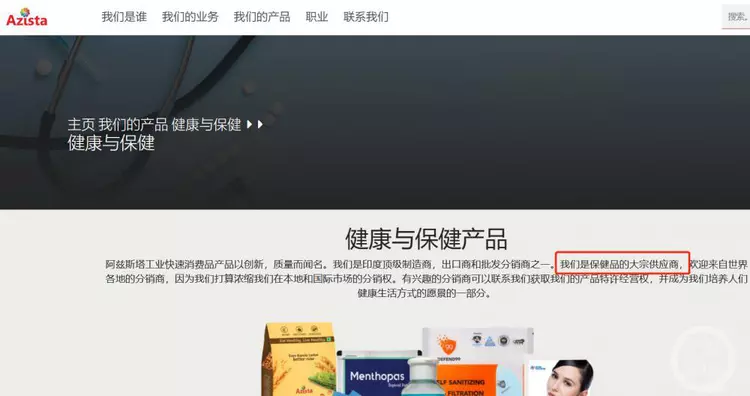
▲The official website of Azista India introduces the company.
On January 9, a senior industry insider in the field of pharmaceuticals in Chongqing told reporters: “From the company’s self-introduction and business, it is not a pharmaceutical company. If the blue box packaging of Paxista is the company’s products, do not exclude the commission processing products, in this case, the approval information in the Indian Drug Administration is important.”
Subsequently, the reporter logged on to the Indian drug regulator, the Central Drugs Standards Control Organization (CDSCO) to conduct a search and found no information related to Azista’s Paxista product packaged in a blue box. The above-mentioned senior industry insider said, “Because of asymmetric information, internet users have higher health risks if they buy such drugs online without permission.”

▲ Introduction of Azista India’s official website.
On January 9, the reporter called the State Drug Administration, and the staff who answered the phone said, “Drugs must be approved by the State Drug Administration before they can be legally produced and operated.” For the market circulation of the green box, blue box of the so-called Indian generic drugs, the above-mentioned staff said, “Consumers have to look to buy this drug there is no State Drug Administration approval number, it is recommended or in the hospital with a doctor’s prescription to buy.”

Average Rating