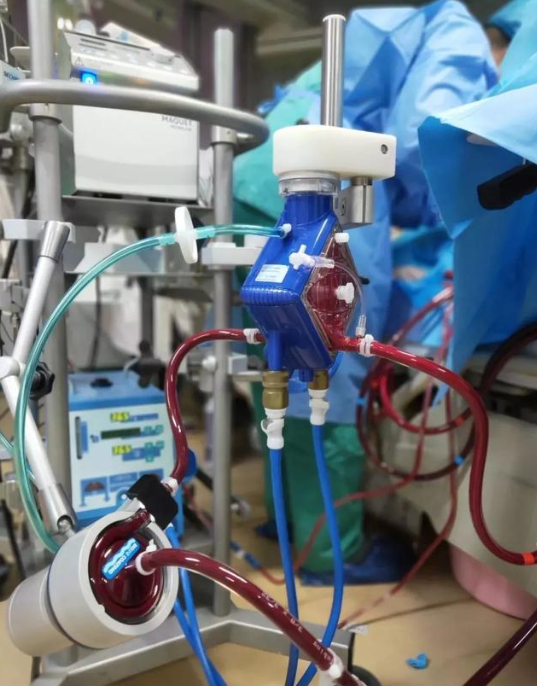
China’s domestic extracorporeal membrane pulmonary oxygenation (ECMO) products were approved for marketing
State Drug Administration website January 5 news, according to the epidemic prevention and control work needs, in order to ensure that the new coronavirus pneumonia seriously ill patients treatment needs, January 4, 2023, the State Drug Administration after review, emergency approval of Shenzhen Hanuo Medical Technology Co., Ltd. extracorporeal cardiopulmonary support auxiliary equipment, single-use membrane oxygenator kit registration application, the two with the use of acute respiratory failure or acute They are used for adult patients with acute respiratory failure or acute cardiopulmonary failure, which are difficult to control by other treatments and have a predictable risk of continuous deterioration or death. As the first domestic ECMO equipment and consumables package, the above products have independent intellectual property rights and the performance indexes basically reach the international level of similar products.
Among them, the extracorporeal cardiopulmonary support auxiliary equipment consists of main unit, pump drive unit, emergency pump drive unit, backup battery, flow bubble sensor, etc. The disposable membrane oxygenator kit consists of membrane oxygenator and arteriovenous line assembly (including centrifugal pump head), pre-filled line assembly, accessory kit assembly and oxygen tubing.
As a salvage treatment device for patients with critical new coronavirus pneumonia for which conventional treatment is ineffective, ECMO is a clear treatment measure in the “New Coronavirus Pneumonia Treatment Plan”. The product will play an important role in meeting the urgent clinical needs, guaranteeing the treatment of patients with the new crown epidemic, and ensuring the implementation of the epidemic prevention and control “health protection and prevention of serious diseases”.
In the product registration process, the State Drug Administration in accordance with the principle of “unified command, early intervention, rapid and efficient, scientific approval”, the establishment of an emergency review working group, responsible for the whole process, guidance, issuing technical review guidelines, increase product registration guidance, speed up the review and approval process, to ensure the safety and effectiveness of On the basis of ensuring the safety and effectiveness of the product to promote the product as soon as possible to meet the urgent needs of the prevention and control of the epidemic.
Drug supervision and management departments will strengthen the post-marketing supervision of the product to protect the safety of patients with machinery.


Average Rating